An Electrochemical Cell Is Based on These Two Half-reactions:
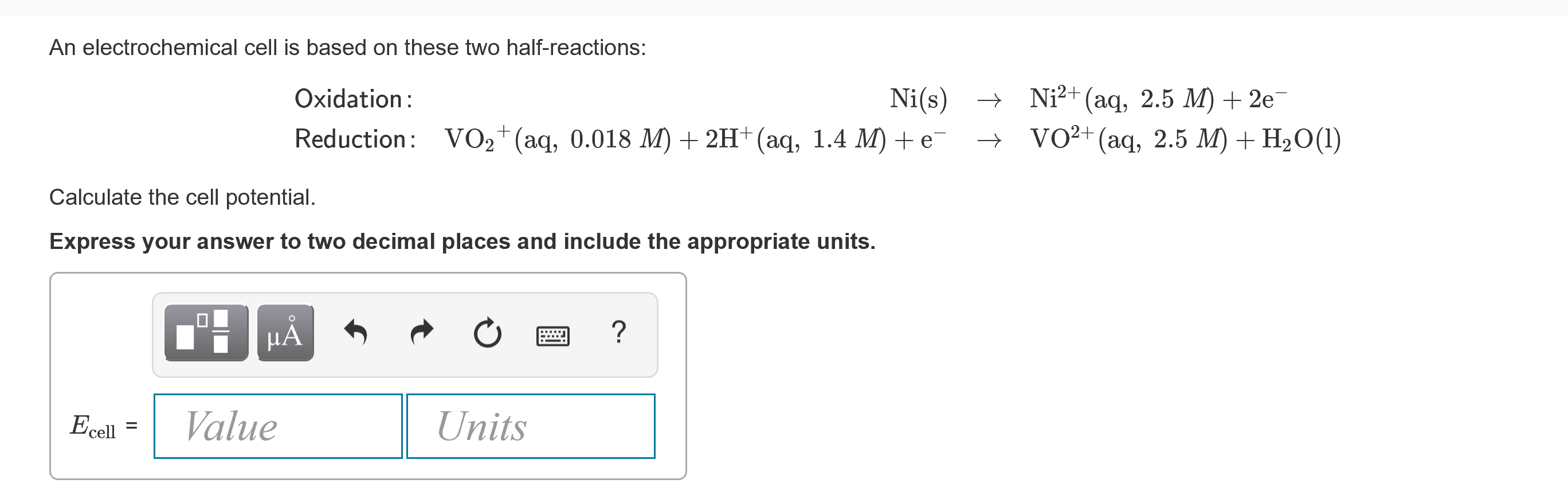
An electrochemical cell is based on these two half-reactionsOxidationReductionNisVO2aq0037M2Haq14MeNi2aq18M2eVO2aq18MH2OlCalculate the cell potential under these nonstandardconcentrations. Sn sSn2 aq 185 M 2e.
Solved An Electrochemical Cell Is Based On These Two Chegg Com
An electrochemical cell is based on these two half-reactions.

. An electrochemical cell is based on these two half-reactions and their given standard reduction potential values. So for a number 75 Im going to calculate the so potential. E 095 V Calculate the cell potential at 25 C.
The first step is to determine the cell potential at its standard state concentrations of 1 molL and pressures of 1 atm at 25C. You can calculate the cell potential for an electrochemical cell from the half-reactions and the operating conditions. An electrochemical cell is based on the following two half-reactions.
Sn s Sn2 aq 200 mol L-12 e E Red. CIO2 g 0270 bar e Cio aq 180 mol L-1 -014 V Eathode 095 V anode Part A Compute the cell potential at 25 C. An electrochemical cell is based on these two half-reactions and their given standard reduction potential values.
Remember that for an. All right So I have to remember this equation pew because the concentra. Pb s Pb2 aq 023 mol L-12 e Eode -013 V Red.
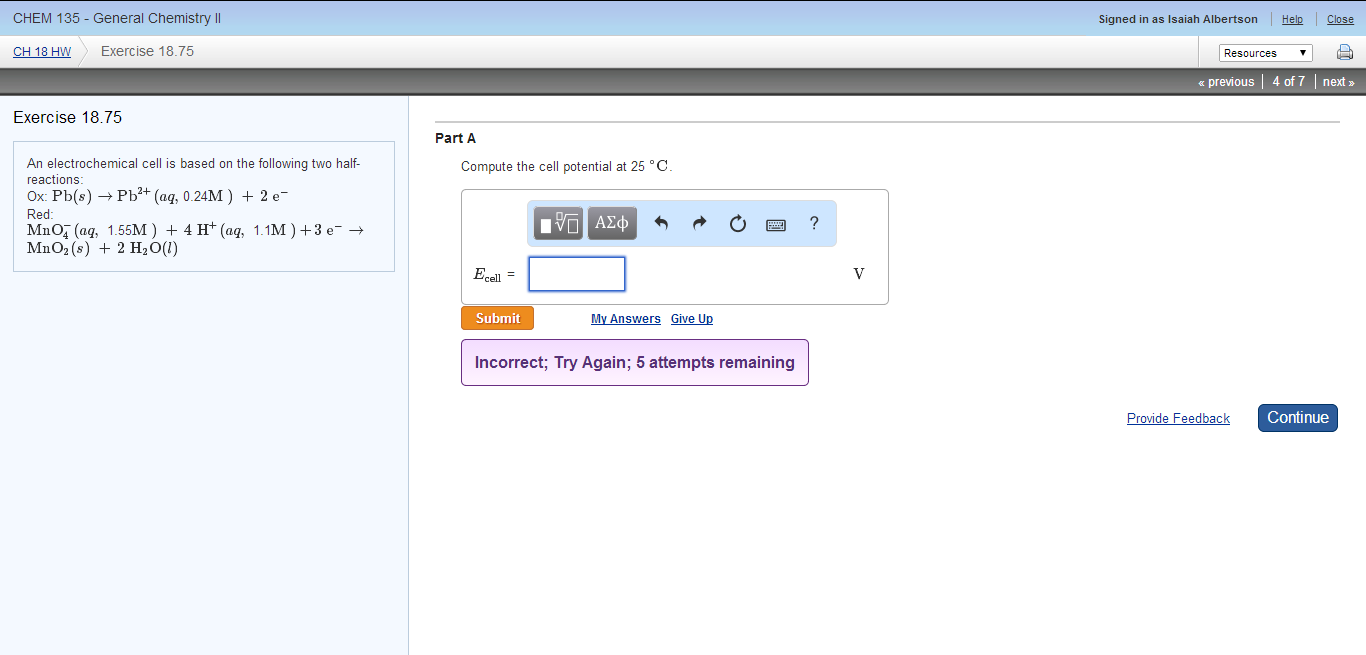
ClO2g0160bareClO2aq165molL1Ecathode095V Part A Compute the cell potential at 25 C. MnO-4aq 170 M 4Haq 24 M 3e- arrow MnO2s 2H2Ol Compute the cell pote. Up to 256 cash back An electrochemical cell is based on these two half-reactions.
MnO2 aq 160 mol L 4H aq 29 mol L-1 3e MnO2 s 2 H20 1 To E cathode 168 V Part A Compute the cell potential at 25C. ClO2g 0100 atm CIO Get the detailed answer. Express your answer to two decimal places and include the appropriate units.
Express the cell potential. ClO2 g0220bareClO2 aq160molL1Ecathode095 V Compute the cell potential at 25 C. ClO2 g 0115bar e ClO2 a q 180molL1 E cathode095V.
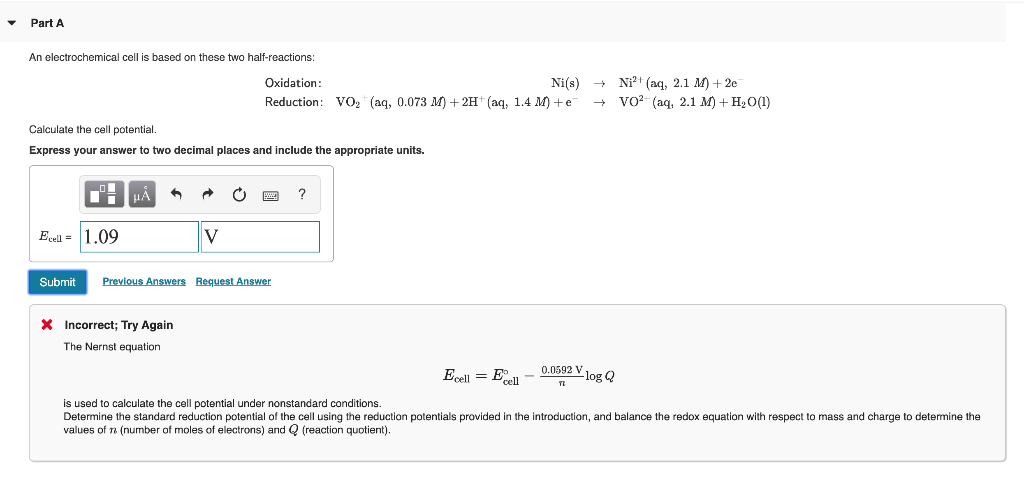
Using formula he said is acquittal standard potential off the cell minus 0592 divided by N look Q he said is the cell potential involves in North Cell. An electrochemical cell is based on these two half-reactions. An electrochemical cell is based on these two half-reactions and their given standard reduction potential values.
C102 9 0220 bar e CIO aq 190 mol L. An electrochemical cell is based on these two half-reactions. MnO4 aq 150 M 4H aq 20 M 3e MnO2s 2 H2O1 Calculate the cell potential at 25C.
Sn s Sn2 aq 174 M 2 e-. Sn s Sn2 a q 175molL12 e E anode014V Red. An electrochemical cell is based on these two half-reactions.
² aq 150 mol L-1 2 e Red. E ClO2 95. NisVO2aq0083M2Haq11MeNi2aq25M2eVO2aq25MH2Ol Calculate the cell potential under these nonstandard concentrations.
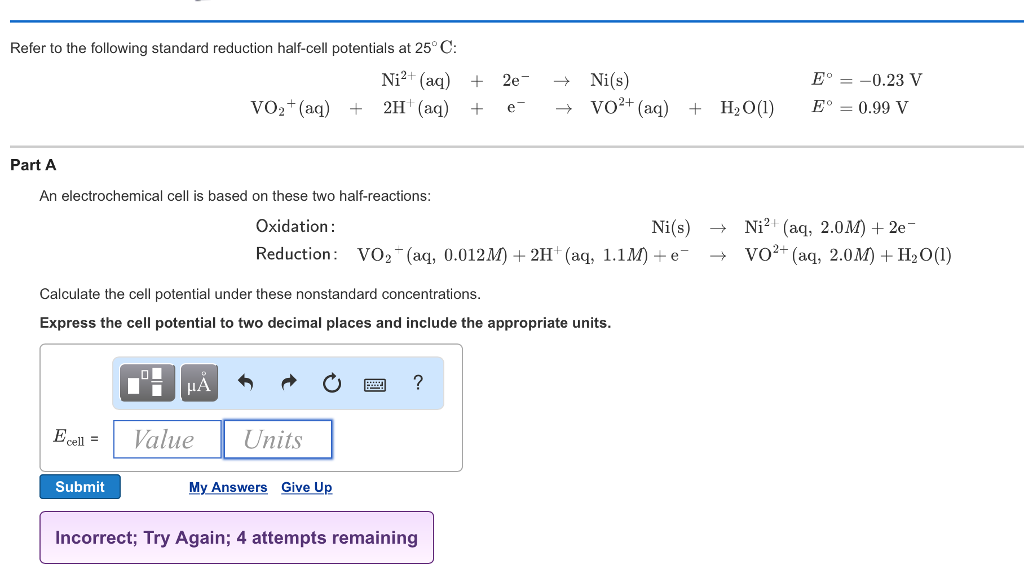
Refer to the following standard reduction half-cell potentials at 25 C. An electrochemical cell is based on these two half-reactions and their given standard reduction potential values. An electrochemical cell is based on these two half-reactions.
Express your answer to two decimal places. An electrochemical cell is based on these two half-reactions. Pbs arrow Pb2aq 023 M 2e- Red.
An electrochemical cell is based on these two half-reactions and their given standard reduction potential values. ClO2 g 0150 atm eClO2 aq 200 M Compute the cell potential at 25 C. Under non standard conditions the cell potential off cell would be calculated.
And is the number off moles off electron transferred and cue is the reaction question substituting. Sn s -- Sn2 aq 160 molL 2e-. SnsSn2aq 185 M 2e Red.
Okay so 25 resource is is to mediate. ClO2g 0150 atm eClO2aq 200 M - 3546492. Sn sSn2 aq160molL12eEanode014 V Red.
Answered expert verified. CIO2 g 0270 bar e ClO aq 180 mol L-. ClO2 g 0145 bar e- -- ClO2- aq 160 molL.
ClO2 g 0270 bar e Cl0 aq 180 mol L Eathode Ox. An electrochemical cell is based on the following two half-reactions. Is the cell potential off standards in.
An electrochemical cell has two half cell reactions as A2 2e- A. Sn s S E anode -014 V 3D 095 V 3D Part A Compute the cell potential at 25 C. An electrochemical cell is based on these two half-reactions.
Oxidation is loss at the anode therefore the oxidation half-reaction occurs in the half-cell containing the anode. E -014 V Red. Sn s Sn² aq 150 mol L-1 2 e E anode -014 V Red.
Sns Sn2 aq 200 M 2e Red. OxidationReductionNisVO2aq0012 M 2Haq11 M eNi2aq20 M 2eVO2aq20 M H2Ol Calculate the cell potential. Eo 034V X X2 2e-.
E SnSn2 -14. Sn s Sn² aq 150 mol L-1 2 e Red. Write the oxidation and reduction half-reactions for the cell.
QuestionAn electrochemical cell is based on these two half. An electrochemical cell is based on the following two half-reactions. Reduction is gain at the cathode so the reduction half-reaction occurs in the half-cell containing the cathode.
Express your answer to two decimal places and include the appropriate units. Eo 237V Caclulate the Ecell. Up to 256 cash back 75.
Pbs Pb2 aq 010 M 2 Red. ClO2 g 0120 atm e- ClO2- aq 144 M Calculate the cell potential at 25C. An electrochemical cell is based on these two half-reactions.
Solved Refer To The Following Standard Reduction Half Cell Chegg Com
Solved An Electrochemical Cell Is Based On The Following Chegg Com
Solved Part A An Electrochemical Cell Is Based On These Two Chegg Com
No comments for "An Electrochemical Cell Is Based on These Two Half-reactions:"
Post a Comment